Abstract
There is an urgent need for a cost-effective, precise, and portable device for rapid and in situ measurement of the critical properties of an emulsion. Here, we report the development of such an optofluidic device for the measurement of mean droplet size ( ) and droplet size distribution (DSD) of a water-in-oil emulsion. We formulated and detected water-in-oil droplets of much smaller dimensions (
) and droplet size distribution (DSD) of a water-in-oil emulsion. We formulated and detected water-in-oil droplets of much smaller dimensions ( ) compared to the detection of larger droplets or plugs (
) compared to the detection of larger droplets or plugs ( to
to  ) reported in the literature, employing a cost effective and portable in-house built optical detection system. Use of the device for the measurement of the frequency of droplets from an on-chip droplet generator is demonstrated and validated using microscopy with excellent accuracy (2%). In addition, we provide some insight into the relatively high uncertainty in the collected signal in case of smaller droplets. The droplet size
) reported in the literature, employing a cost effective and portable in-house built optical detection system. Use of the device for the measurement of the frequency of droplets from an on-chip droplet generator is demonstrated and validated using microscopy with excellent accuracy (2%). In addition, we provide some insight into the relatively high uncertainty in the collected signal in case of smaller droplets. The droplet size  is characterized in terms of forward scatter signal
is characterized in terms of forward scatter signal  and residence time
and residence time  . We further argue that normalized residence time
. We further argue that normalized residence time  of droplets in the detection zone which correlates linearly with droplet size
of droplets in the detection zone which correlates linearly with droplet size  is a better parameter to measure droplet size
is a better parameter to measure droplet size  , compared to the forward scatter signal
, compared to the forward scatter signal  which correlates nonlinearly with
which correlates nonlinearly with  . Finally, the device is used to count the number of droplets of different size to predict
. Finally, the device is used to count the number of droplets of different size to predict  and DSD of emulsions. The results were compared with that obtained from traditional microscopy and a very good match (10–13%) was found, in contrast to previously reported non-portable off-chip methods that are 20–44% accurate. Thus, the reported device possesses high potential for accurate measurement of
and DSD of emulsions. The results were compared with that obtained from traditional microscopy and a very good match (10–13%) was found, in contrast to previously reported non-portable off-chip methods that are 20–44% accurate. Thus, the reported device possesses high potential for accurate measurement of  and DSD of emulsions in practical applications.
and DSD of emulsions in practical applications.
Export citation and abstract BibTeX RIS
1. Introduction
Emulsions are liquid droplets or crystals dispersed in another liquid of different polarity with a thin film of surfactant at the interface for stabilization [1–3]. Due to the distinctive properties including high surface area-to-volume ratio, increased bioavailability, monodispersity, increased optical transparency, and novel rheology, both water-in-oil (W/O) and oil-in-water (O/W) emulsions are being widely studied [1, 3–10]. Emulsions have applications in various industries including agriculture [1, 10, 11], pharmaceutical [12–14], health and skin care [6, 15], polymerization [16–18] and food [19, 20]. Mean droplet size ( ) and droplet size distribution (DSD) are the two most important characteristics of emulsions [19, 21] which can affect the stability (i.e. avoiding coalescence or Ostwald ripening) and reproducibility of emulsions [19].
) and droplet size distribution (DSD) are the two most important characteristics of emulsions [19, 21] which can affect the stability (i.e. avoiding coalescence or Ostwald ripening) and reproducibility of emulsions [19].
Measurement of mean droplet size and DSD without altering the emulsion itself is challenging, which limits the understanding of the formation and degradation of emulsions [21]. Currently, dynamic light scattering [22], surfactant titration [21], automated scanning electron microscopy [23], acoustic attenuation spectroscopy [23, 24], capillary hydrodynamic fractionation [25], and optical microscopy [19, 26] are employed for the measurement. Most of these techniques are characterized for measurement of non-deformable objects, while droplet emulsions are deformable and thus such techniques are not reliable for the measurement of  and DSD of emulsions [21]. In addition, some of these techniques require sample preparation which alters the DSD before the measurements [21]. Also, most of these techniques require complicated and expensive instruments and thus are only available at central facilities, which further limit their accessibility.
and DSD of emulsions [21]. In addition, some of these techniques require sample preparation which alters the DSD before the measurements [21]. Also, most of these techniques require complicated and expensive instruments and thus are only available at central facilities, which further limit their accessibility.
Emulsions are devised either by using high energy or low energy methods. High energy methods use externally applied high shear to surpass interfacial tension and viscous forces to form emulsions [27], while low energy methods utilize the unique characteristics of oil, water, and emulsifier to formulate emulsions [4]. In recent years, a high energy method, micro-fluidization, has been used as one of the most efficient techniques for generating emulsions, since the droplets devised by this method are smaller and exhibit narrower size distribution [4, 28, 29]. In a microfluidizer, a coarse emulsion is infused and divided into two jets. These jets are passed through microchannels towards an impingement region where they collide at high pressure to devise homogenized emulsions [4, 29]. Recently, a dual-channel micro-fluidization method was employed which does not require a coarse emulsion, and thus the concentration of phases can be controlled in real time. The emulsion was finally collected at the output reservoir for  and DSD measurements and further analysis [4]. Thus, a device for in situ measurement of mean droplet size
and DSD measurements and further analysis [4]. Thus, a device for in situ measurement of mean droplet size  and DSD is imperative for real time control of these parameters during the formulation of emulsions.
and DSD is imperative for real time control of these parameters during the formulation of emulsions.
Optofluidics is an emerging field wherein light and fluids are manipulated in synergy to develop novel tools and techniques for devising either tunable optical components or lab-on-chip bio-chemical sensors with enhanced sensitivity and adaptability [30, 31]. Several optofluidic lab-on-chip devices have been implemented in recent years for various applications including single cell analysis [32], controlling liquid motion using light [33], sunlight based fuel-production [34], microfabrication [35] and flow cytometry [36]. In particular the microflow cytometry setup has been used for different applications including counting and studying biological cells [37], bacteria [38], cellular deoxyribonucleic acid (DNA) [39, 40] and droplets [41, 42].
Here, we report the development and characterization of an optofluidic device for in situ measurement of mean droplet size  and DSD of an emulsion. First, we studied the size
and DSD of an emulsion. First, we studied the size  and frequency
and frequency  of droplets generated using a microfluidic droplet generator in terms of the scattered signal
of droplets generated using a microfluidic droplet generator in terms of the scattered signal  and residence time
and residence time  . Droplets of dimensions as small as
. Droplets of dimensions as small as  are generated and detected using the proposed device, which is in clear contrast with the detection of droplets of size
are generated and detected using the proposed device, which is in clear contrast with the detection of droplets of size  –
– reported in literature [41]. For bigger droplets, we depict that the advancing and receding edges of a droplet resulted in two peaks within a single pulse and as it becomes smaller, only one peak is observed. Droplets align themselves to the center of the microchannel due to the deformability induced lift forces [43], however, the time required for the migration toward the centerline is proportional to the size of the droplets. This is responsible for the large uncertainty in the signal observed in case of smaller droplets, which is discussed in detail with the help of scatter plot. The droplet size was correlated with the scattered signal
reported in literature [41]. For bigger droplets, we depict that the advancing and receding edges of a droplet resulted in two peaks within a single pulse and as it becomes smaller, only one peak is observed. Droplets align themselves to the center of the microchannel due to the deformability induced lift forces [43], however, the time required for the migration toward the centerline is proportional to the size of the droplets. This is responsible for the large uncertainty in the signal observed in case of smaller droplets, which is discussed in detail with the help of scatter plot. The droplet size was correlated with the scattered signal  and normalized residence time
and normalized residence time  . We report that the normalized residence time
. We report that the normalized residence time  is a better parameter to measure droplet size, since the correlation is linear in comparison to the scattered signal which gives nonlinear correlation as droplet size increases. Further, normalized residence time is also independent of velocity of the fluid in the microchannel, making it widely applicable. The correlation was used further to predict mean droplet size
is a better parameter to measure droplet size, since the correlation is linear in comparison to the scattered signal which gives nonlinear correlation as droplet size increases. Further, normalized residence time is also independent of velocity of the fluid in the microchannel, making it widely applicable. The correlation was used further to predict mean droplet size  and DSD of W/O emulsion. The predictions were compared with the data obtained using optical microscopy, which match very well within a maximum error of 10% in contrast to the 20% and 44% difference in
and DSD of W/O emulsion. The predictions were compared with the data obtained using optical microscopy, which match very well within a maximum error of 10% in contrast to the 20% and 44% difference in  and DSD respectively between the AAS and microscopy data reported in the literature [23]. The proposed device shows improvements over previously reported macro methods due to the self-alignment of droplets, which minimizes the uncertainty in the signal and thus improves the accuracy and sensitivity of the system.
and DSD respectively between the AAS and microscopy data reported in the literature [23]. The proposed device shows improvements over previously reported macro methods due to the self-alignment of droplets, which minimizes the uncertainty in the signal and thus improves the accuracy and sensitivity of the system.
2. Experiments
2.1. Device design and setup
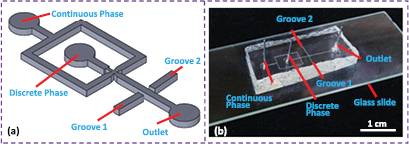
Figures 1(a) and (b), respectively, show the schematic and image of the device used for predicting droplet size and frequency by measuring the forward scattered signal (FSC). The device was also used for measuring mean droplet size ( ) and DSD of emulsions. The device is equipped with grooves to insert optical fibers. As shown, an optical fiber in Groove 1 was embedded to generate a focused illumination beam (of size ~10 µm). In order to avoid saturation of the photodiode due to direct exposure of the incident laser beam, the collection fiber in Groove 2 was placed
) and DSD of emulsions. The device is equipped with grooves to insert optical fibers. As shown, an optical fiber in Groove 1 was embedded to generate a focused illumination beam (of size ~10 µm). In order to avoid saturation of the photodiode due to direct exposure of the incident laser beam, the collection fiber in Groove 2 was placed  anticlockwise with respect to the incident beam, and was used to obtain FSC. This design constraint is also consistent with the literature [43], which reports collection of FSC at
anticlockwise with respect to the incident beam, and was used to obtain FSC. This design constraint is also consistent with the literature [43], which reports collection of FSC at  –
– with respect to the incident beam axis [43, 44].The PDMS microchannel device was fabricated using a standard photolithography technique followed by a soft lithography process, a detailed description of which is provided in our previous work [45]. The device includes an on-chip droplet generator to generate droplets of deionized (DI) water as discrete phase in oil as continuous phase. In our experiments with emulsions, the continuous W/O emulsion was introduced through the discrete phase inlet and oil was used to space out the droplets. The depth and width of the fiber channels were kept as
with respect to the incident beam axis [43, 44].The PDMS microchannel device was fabricated using a standard photolithography technique followed by a soft lithography process, a detailed description of which is provided in our previous work [45]. The device includes an on-chip droplet generator to generate droplets of deionized (DI) water as discrete phase in oil as continuous phase. In our experiments with emulsions, the continuous W/O emulsion was introduced through the discrete phase inlet and oil was used to space out the droplets. The depth and width of the fiber channels were kept as  , so that the standard available
, so that the standard available  single mode and
single mode and  multimode fibers can be inserted into the grooves. The fiber grooves were filled with index matching liquid (Fiber Instruments Sales Inc., USA) to minimize reflection losses. The width of the main fluid channel was kept as 100 µm and the width of the flow focusing junction was kept as 30 µm. The depth of the fluid channel is kept as 130 µm throughout. The droplets self-migrate towards the center of the channel [43] due to deformability induced lift force acting on them.
multimode fibers can be inserted into the grooves. The fiber grooves were filled with index matching liquid (Fiber Instruments Sales Inc., USA) to minimize reflection losses. The width of the main fluid channel was kept as 100 µm and the width of the flow focusing junction was kept as 30 µm. The depth of the fluid channel is kept as 130 µm throughout. The droplets self-migrate towards the center of the channel [43] due to deformability induced lift force acting on them.
Figure 1. (a) Schematic of the device and (b) image of the device used for measuring FSC and frequency of droplets.
Download figure:
Standard image High-resolution image2.2. Materials and methods
Mineral oil (Sigma Aldrich, Bangalore, India) was filtered using a  polytetrafluoroethylene (PTFE) filter (Axiva Sichem Biotech, Chennai, India) to avoid the device clogging. The kinematic viscosity of the mineral oil was
polytetrafluoroethylene (PTFE) filter (Axiva Sichem Biotech, Chennai, India) to avoid the device clogging. The kinematic viscosity of the mineral oil was  . To increase the stability of droplets, 5% wt/wt of surfactant Span 85 (Sigma Aldrich Bangalore, India) was added to mineral oil. DI water was filtered separately using a nylon
. To increase the stability of droplets, 5% wt/wt of surfactant Span 85 (Sigma Aldrich Bangalore, India) was added to mineral oil. DI water was filtered separately using a nylon  filter (Axiva Sichem Biotech, Chennai, India) and
filter (Axiva Sichem Biotech, Chennai, India) and  wt/wt of surfactant Tween 80 was added to prevent the sedimentation of water droplets in oil. Water was used as the discrete phase while oil was used as the continuous phase. The mean droplet size
wt/wt of surfactant Tween 80 was added to prevent the sedimentation of water droplets in oil. Water was used as the discrete phase while oil was used as the continuous phase. The mean droplet size  of an emulsion is directly proportional to the concentration of the discrete phase in the continuous phase [4]. W/O emulsions of different mean droplet size
of an emulsion is directly proportional to the concentration of the discrete phase in the continuous phase [4]. W/O emulsions of different mean droplet size  were obtained by varying the concentration (% volume) of dispersed phase (water) in the continuous phase (oil). DI water of volumes
were obtained by varying the concentration (% volume) of dispersed phase (water) in the continuous phase (oil). DI water of volumes  ,
,  ,
,  , and
, and  were separately mixed with fixed volume (
were separately mixed with fixed volume ( of oil using a vortex mixer for 1.0 s, resulting in
of oil using a vortex mixer for 1.0 s, resulting in  ,
,  ,
,  , and
, and  of W/O emulsions.
of W/O emulsions.
2.3. Optical detection system
Sensing of droplets and measurement of DSD in microchannels have been achieved using various optical interrogation techniques, for instance forward scattering [42], surface enhanced raman scattering [46], fluorescence [40, 41], and optical spectroscopy [47]. However, expensive, high power, potentially hazardous laser sources in the visible range (400 nm–650 nm) were used in these works. In contrast, in the present work, a cost effective, low power (1.0 mW), and non-hazardous [48] laser source near infrared range (1550 nm) was used for the sample illumination. In the previous works, a range of complicated and expensive optical detection systems including microscope coupled spectrometer [46], photon multiplier tubes (PMT) [40], and avalanche photodiode (APD) [42] were used for the measurements. In contrast, in the present work, we have used portable and cost-effective InGaAs photodiode for the detection.
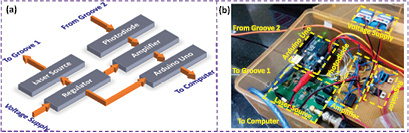
Figures 2(a) and (b), respectively, show a block diagram and image of the in-house built optical detection system used for the measurements. A single mode ( ) fiber coupled
) fiber coupled  ,
,  laser diode was used for illumination. The forward scatter (FSC) data was collected with multimode (
laser diode was used for illumination. The forward scatter (FSC) data was collected with multimode ( ) fiber coupled InGaAs photodiode (Epitaxx Inc, Princeton, NJ). The scattered signal was amplified with a trans-impedance amplifier (TL084, Texas Instruments Inc., USA) before converting to digital signal using a microcontroller (Arduino Uno R3, Arduino, Italy). The data was serially transmitted to the terminal with the baud rate of 2.5 × 105 bits per second (bps). Data was analyzed on a personal computer (PC) using a Python code to obtain the residence time and scattered signal amplitude of each particle crossing the detection region (i.e. focused beam spot). A voltage regulator circuit was employed to obtain regulated voltage of
) fiber coupled InGaAs photodiode (Epitaxx Inc, Princeton, NJ). The scattered signal was amplified with a trans-impedance amplifier (TL084, Texas Instruments Inc., USA) before converting to digital signal using a microcontroller (Arduino Uno R3, Arduino, Italy). The data was serially transmitted to the terminal with the baud rate of 2.5 × 105 bits per second (bps). Data was analyzed on a personal computer (PC) using a Python code to obtain the residence time and scattered signal amplitude of each particle crossing the detection region (i.e. focused beam spot). A voltage regulator circuit was employed to obtain regulated voltage of  V to supply power to laser source, trans-impedance amplifier, and microcontroller circuits.
V to supply power to laser source, trans-impedance amplifier, and microcontroller circuits.
Figure 2. (a) Block diagram; (b) image of the optical detection system that was built and used in our experiments.
Download figure:
Standard image High-resolution image2.4. Experimental setup
Syringe pumps (Kent Scientific Corporation, USA) were used to infuse the fluids into the microchannels. An inverted microscope (Axiovert A1, Carl Ziess GmbH, Gemany) coupled with high speed camera (SA3, Phtoron, USA) was used to capture the videos at 3000 frames per second to accurately capture the droplets. Images and videos were analyzed using ImageJ (Rasband, W. S., Image J, USA) to obtain the droplet generation rate ( ), and droplet diameter (
), and droplet diameter ( ). Mean droplet size (
). Mean droplet size ( ) and DSD of the droplets were quantified with improved Naeubaer Haemocytometer (Marienfeld, Germany). Emulsion of volume
) and DSD of the droplets were quantified with improved Naeubaer Haemocytometer (Marienfeld, Germany). Emulsion of volume  was spread on a haemocytometer and images were captured using the inverted microscope. MATLAB (The Math Works Inc., USA) was used to process the images to obtain
was spread on a haemocytometer and images were captured using the inverted microscope. MATLAB (The Math Works Inc., USA) was used to process the images to obtain  and DSD.
and DSD.
3. Results and discussion
3.1. Droplet size and frequency measurement
Continuous stream of droplets was obtained by infusing water (discrete phase) and oil (continuous phase) into the microchannel at flow rates  and
and  respectively. The percentage volume fraction of water
respectively. The percentage volume fraction of water  , defined as
, defined as  , was varied between 3 to 12%. The size of the generated droplets
, was varied between 3 to 12%. The size of the generated droplets  was controlled by changing the continuous to discrete phase flow rate ratio
was controlled by changing the continuous to discrete phase flow rate ratio  , maintaining fluid flow velocity in the range of 3.5 mm s−1 to 5.0 mm s−1 in the microchannel. This corresponds to the variation of Reynolds number (
, maintaining fluid flow velocity in the range of 3.5 mm s−1 to 5.0 mm s−1 in the microchannel. This corresponds to the variation of Reynolds number ( ) in the range of 0.022–0.032, where
) in the range of 0.022–0.032, where  is the flow velocity,
is the flow velocity,  is the hydraulic diameter of the channel and
is the hydraulic diameter of the channel and  is the kinematic viscosity. At such low Reynolds number (
is the kinematic viscosity. At such low Reynolds number ( ), due to the deformability induced lift forces [43] and symmetric flow conditions in the microchannel, the droplets tend to move to the region with zero shear rate and thus self-align themselves at the centerline of the microchannel. Thus droplets are self-focused in both the horizontal and vertical directions. The stream of droplets was interrogated with a beam of laser light (1550 nm, 1.0 mW) and detected with the in-house built optical detection system explained in section 2.3. When a droplet traverses the detection region, it obstructs the passage of the laser beam, which results in a pulse in the detected signal as observed in figure 3. An in-house software was built using Python to serially receive the data at
), due to the deformability induced lift forces [43] and symmetric flow conditions in the microchannel, the droplets tend to move to the region with zero shear rate and thus self-align themselves at the centerline of the microchannel. Thus droplets are self-focused in both the horizontal and vertical directions. The stream of droplets was interrogated with a beam of laser light (1550 nm, 1.0 mW) and detected with the in-house built optical detection system explained in section 2.3. When a droplet traverses the detection region, it obstructs the passage of the laser beam, which results in a pulse in the detected signal as observed in figure 3. An in-house software was built using Python to serially receive the data at  bps. The data was passed through a moving average filter to remove the high frequency and random noise. The software program senses a droplet in the microchannel by detecting the presence of a pulse in the data. The width of the pulse corresponds to the time required by the droplet to cross the detection zone (residence time
bps. The data was passed through a moving average filter to remove the high frequency and random noise. The software program senses a droplet in the microchannel by detecting the presence of a pulse in the data. The width of the pulse corresponds to the time required by the droplet to cross the detection zone (residence time  ), whereas the amplitude of the detected pulse denotes the scattered signal amplitude (
), whereas the amplitude of the detected pulse denotes the scattered signal amplitude ( ). Figures 3(a)–(c) show the experimental images of the droplets and data captured using the optical detection system at continuous to discrete phase flow rate ratio (i.e.
). Figures 3(a)–(c) show the experimental images of the droplets and data captured using the optical detection system at continuous to discrete phase flow rate ratio (i.e.  )
)  ,
,  , and
, and  , respectively. It can be observed from figure 3 that as flow rate ratio (
, respectively. It can be observed from figure 3 that as flow rate ratio ( ) increases, the size of the droplets
) increases, the size of the droplets  decreases and correspondingly the pulse signal reduces in amplitude (
decreases and correspondingly the pulse signal reduces in amplitude ( ) and duration (
) and duration ( ).
).
Figure 3. Experimental images and data captured at flow rate ratio (a) f = 7 (b) f = 20 (c) f = 25.
Download figure:
Standard image High-resolution imageFor bigger droplets ( in figure 3(a)), the advancing and receding edges of a droplet resulted in two peaks within a single pulse, as shown in figure 3(a). This observation is consistent with the work reported previously on the detection of droplets of comparable sizes [42]. However, as the droplet becomes smaller, only one peak was observed in a pulse as shown in figures 3(b) and (c), this is attributed to the fact that as the size of the droplet reduces, curvature of the droplet increases significantly and peaks approach each other, finally resulting in a single peak for a given droplet.
in figure 3(a)), the advancing and receding edges of a droplet resulted in two peaks within a single pulse, as shown in figure 3(a). This observation is consistent with the work reported previously on the detection of droplets of comparable sizes [42]. However, as the droplet becomes smaller, only one peak was observed in a pulse as shown in figures 3(b) and (c), this is attributed to the fact that as the size of the droplet reduces, curvature of the droplet increases significantly and peaks approach each other, finally resulting in a single peak for a given droplet.
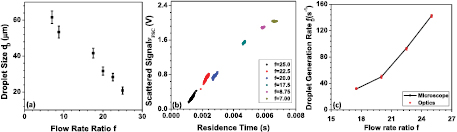
Images captured from optical microscopy were analysed using ImageJ to determine exact size of the droplets  . Figure 4(a) shows the mean droplet size at various flow rate ratios
. Figure 4(a) shows the mean droplet size at various flow rate ratios  . It is observed that as the flow rate ratio
. It is observed that as the flow rate ratio  increases, the size of droplets
increases, the size of droplets  decreases. The error bar in the plot shows the standard deviation of the data. Figure 4(b) represents the scatter plot of FSC
decreases. The error bar in the plot shows the standard deviation of the data. Figure 4(b) represents the scatter plot of FSC  versus residence time
versus residence time  of the droplets at various flow rate ratios
of the droplets at various flow rate ratios  . The results clearly show that as the flow rate ratio
. The results clearly show that as the flow rate ratio  increases from
increases from  to
to  i.e. as the size of droplets decreases, the strength of both the scattered signal
i.e. as the size of droplets decreases, the strength of both the scattered signal  and residence time
and residence time  decreases. Consequently, we observe distinct colonies on the scatter plot depending on the flow rate ratio or the resulting droplet size. The different colonies observed on the plot also indicate that the droplets align themselves at the center of the channel and pass through the detection region single-file. For a given flow rate ratio
decreases. Consequently, we observe distinct colonies on the scatter plot depending on the flow rate ratio or the resulting droplet size. The different colonies observed on the plot also indicate that the droplets align themselves at the center of the channel and pass through the detection region single-file. For a given flow rate ratio  or the corresponding droplet size
or the corresponding droplet size  , the small variation in residence time indicates that the droplets are focused and thus travel at the same velocity. Similarly, the small variation in the forward scatter signal
, the small variation in residence time indicates that the droplets are focused and thus travel at the same velocity. Similarly, the small variation in the forward scatter signal  indicates that the droplets travel single-file. From figure 4(b), it is observed that the spread in the scattered signal
indicates that the droplets travel single-file. From figure 4(b), it is observed that the spread in the scattered signal  and residence time
and residence time  is greater at higher flow rate ratios
is greater at higher flow rate ratios  (i.e. for smaller droplets). This can be attributed to the fact that due to lower migration velocity [43], the smaller droplets may not get focused at the center of the channel and may be present at different locations from the center. As a result, a larger variation in the FSC signal
(i.e. for smaller droplets). This can be attributed to the fact that due to lower migration velocity [43], the smaller droplets may not get focused at the center of the channel and may be present at different locations from the center. As a result, a larger variation in the FSC signal  and residence time
and residence time  is oberserved in the case of smaller droplets in comparison to bigger droplets (which get focused at the center).
is oberserved in the case of smaller droplets in comparison to bigger droplets (which get focused at the center).
Figure 4. (a) Droplet size  at different flow rate ratio
at different flow rate ratio  ; (b) scattered signal
; (b) scattered signal  versus residence time
versus residence time  at various flow rate ratio
at various flow rate ratio  ; (c) comparison of droplet frequency
; (c) comparison of droplet frequency  rate at different flow rate ratio
rate at different flow rate ratio  using the microscope and optofluidic system.
using the microscope and optofluidic system.
Download figure:
Standard image High-resolution imageThe previous literature [49] extensively reports the formulation and sensing of droplets of a size comparable to that of the microfluidic channels (such droplets are also called plugs). In the present work, a much lower volume fraction of water  = 3% was used to generate and detect monodispersed droplets of size as small as
= 3% was used to generate and detect monodispersed droplets of size as small as  , in contrast to the higher
, in contrast to the higher  in the range 10%–60% used in the works reported in the literature [40, 42, 46, 47]. The size of the plugs generated and detected previously vary from
in the range 10%–60% used in the works reported in the literature [40, 42, 46, 47]. The size of the plugs generated and detected previously vary from  to
to  [41]. Furthermore, we reported the scatter plot of forward scatter signal
[41]. Furthermore, we reported the scatter plot of forward scatter signal  versus the residence time
versus the residence time  for droplets of different sizes and provide some insight on the large variations in the signal in case of smaller droplets.
for droplets of different sizes and provide some insight on the large variations in the signal in case of smaller droplets.
The device was also employed for the measurement of droplet generation rate ( ). The time gap between two consecutive droplets
). The time gap between two consecutive droplets  to cross the detection region was measured and the droplet generation rate
to cross the detection region was measured and the droplet generation rate  was calculated as the reciprocal of
was calculated as the reciprocal of  . The videos captured using the microscope were analysed to determine the droplet generation rate
. The videos captured using the microscope were analysed to determine the droplet generation rate  and compared with that obtained from the optical detection system as shown in figure 4(c). The droplet generation rate
and compared with that obtained from the optical detection system as shown in figure 4(c). The droplet generation rate  was determined for each pair of consecutive droplets and the mean of the data is taken. The error bar in figure 4(c) corresponds to the standard deviation of data. An excellent match (within a maximum error of 2%) between the data obtained from microscopy and the optofluidic device is observed. Thus the proposed device can be used as a droplet counter.
was determined for each pair of consecutive droplets and the mean of the data is taken. The error bar in figure 4(c) corresponds to the standard deviation of data. An excellent match (within a maximum error of 2%) between the data obtained from microscopy and the optofluidic device is observed. Thus the proposed device can be used as a droplet counter.
3.2. Characterization of optofluidic device
The variation in the mean of the scattered signal  and the normalized residence time
and the normalized residence time  with droplet size
with droplet size  is depicted in figures 5(a) and (b) respectively. In both the plots, error bars represent the standard deviation of the data. In figure 5(b), residence time of the droplet
is depicted in figures 5(a) and (b) respectively. In both the plots, error bars represent the standard deviation of the data. In figure 5(b), residence time of the droplet  is normalized with time scale of the device i.e.
is normalized with time scale of the device i.e.  , where
, where  is width of the microchannel and
is width of the microchannel and  is the continuous phase velocity in the microchannel, since residence time depends on superficial velocity
is the continuous phase velocity in the microchannel, since residence time depends on superficial velocity  of the fluid and is proprtional to total flow rate (
of the fluid and is proprtional to total flow rate ( ). Figure 5(a) shows that the the strength of the scattered signal
). Figure 5(a) shows that the the strength of the scattered signal  increases linearly for smaller droplets but becomes nonlinear at higher droplet size. This characteristic can be attributed to the fact that the strength of scattered signal
increases linearly for smaller droplets but becomes nonlinear at higher droplet size. This characteristic can be attributed to the fact that the strength of scattered signal  would saturate as droplet size approaches the size of microchannel (
would saturate as droplet size approaches the size of microchannel ( in the present device). At such droplet sizes, the droplets become deformed and the lateral dimension (normal to flow direction) of the droplet which contributes to the forward scatter does not change any further. From figure 5(b), it is observed that the value of residence time
in the present device). At such droplet sizes, the droplets become deformed and the lateral dimension (normal to flow direction) of the droplet which contributes to the forward scatter does not change any further. From figure 5(b), it is observed that the value of residence time  increases linearly with the size of the droplet. This is explained by the fact that the residence time depends on the droplet size along the flow direction, which increases proportionately with increase in the droplet size. Thus we find that the normalized residence time
increases linearly with the size of the droplet. This is explained by the fact that the residence time depends on the droplet size along the flow direction, which increases proportionately with increase in the droplet size. Thus we find that the normalized residence time  , which exhibits linear charcteristics, is a better parameter to characterize the size of droplets. However, other properties including refractive index and granularity can be charcterized using scattered signals [44]. Here, the scattered signal
, which exhibits linear charcteristics, is a better parameter to characterize the size of droplets. However, other properties including refractive index and granularity can be charcterized using scattered signals [44]. Here, the scattered signal  was fitted to a quadratic polynomial fitted with
was fitted to a quadratic polynomial fitted with  value of 0.983, wheras residence time
value of 0.983, wheras residence time  was fitted to a linear curve with the
was fitted to a linear curve with the  value of 0.998. The droplet size was correlated with the residence time as follows:
value of 0.998. The droplet size was correlated with the residence time as follows:  , where the value of
, where the value of  is found to be
is found to be  . This correlation was further used in the prediction of droplet size in case of emulsions.
. This correlation was further used in the prediction of droplet size in case of emulsions.
Figure 5. (a) Scattered signal  for varying droplet sizes
for varying droplet sizes  ; (b) normalized residence time
; (b) normalized residence time  for varying droplet sizes
for varying droplet sizes  .
.
Download figure:
Standard image High-resolution imageIn the literature, the residence time  of droplets or the pulse width of signal was used to represent the size of droplets
of droplets or the pulse width of signal was used to represent the size of droplets  [42]. However, the residence time
[42]. However, the residence time  could vary with the variation in the flow rate in a microchannel (
could vary with the variation in the flow rate in a microchannel ( ), thus it is a very intricate process of measurement to gain the absolute size of droplets unless the flow velocity is kept fixed [46]. Here, we normalize the residence time
), thus it is a very intricate process of measurement to gain the absolute size of droplets unless the flow velocity is kept fixed [46]. Here, we normalize the residence time  with superficial velocity of the fluid and correlate the normalized residence time
with superficial velocity of the fluid and correlate the normalized residence time  with droplet size
with droplet size  to eliminate the effect of fluid velocity on the measurement. Similarly, a correlation between amplitude of the forward scatter signal
to eliminate the effect of fluid velocity on the measurement. Similarly, a correlation between amplitude of the forward scatter signal  with droplet size
with droplet size  was studied [41]. However, we conclude that the normalized residence time
was studied [41]. However, we conclude that the normalized residence time  is a better parameter for predicting
is a better parameter for predicting  due to its linear correlation with
due to its linear correlation with  whereas
whereas  correlates nonlinearly with
correlates nonlinearly with  .
.
3.3. Measurement of size distribution of an emulsion
Experiments were performed using emulsions with varying volume fraction of water  between 1.25%–5%, formulated as explained in section 2.2. The volume fraction of water used in the emulsions is consistent with that used for the droplet generation experiments presented in section 3.1. Mineral oil was used as a sheath fluid to space out the droplets, to avoid a large number passing the detection region simultaneously. The emulsions were formulated as explained in section 2.2 and used as a sample for carrying out the measurements. When each droplet crosses the detection region, the forward scattered signal
between 1.25%–5%, formulated as explained in section 2.2. The volume fraction of water used in the emulsions is consistent with that used for the droplet generation experiments presented in section 3.1. Mineral oil was used as a sheath fluid to space out the droplets, to avoid a large number passing the detection region simultaneously. The emulsions were formulated as explained in section 2.2 and used as a sample for carrying out the measurements. When each droplet crosses the detection region, the forward scattered signal  and normalized residence time
and normalized residence time  of each event was collected. From the measured normalized residence time
of each event was collected. From the measured normalized residence time  , the droplet size
, the droplet size  of each event was predicted using the correlation presented in section 3.2. The predicted droplet size was further compared with the results obtained using microscopy. Using the optofluidic device, the mean droplet size
of each event was predicted using the correlation presented in section 3.2. The predicted droplet size was further compared with the results obtained using microscopy. Using the optofluidic device, the mean droplet size  of each emulsion was determined from a large number of events crossing the detection region. Emulsion of volume
of each emulsion was determined from a large number of events crossing the detection region. Emulsion of volume  was dispensed on a haemocytometer and images were captured using optical microscopy at 20x magnification. A MATLAB image processing toolbox was used to calculate the mean droplet size
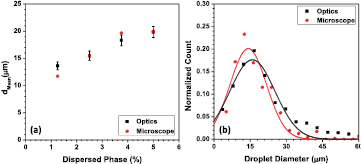
was dispensed on a haemocytometer and images were captured using optical microscopy at 20x magnification. A MATLAB image processing toolbox was used to calculate the mean droplet size  of the emulsion. Figure 6(a) presents
of the emulsion. Figure 6(a) presents  of emulsions prepared with different percentage of dispersed phase. The data obtained from the optofluidic device and predicted by the correlation is in good agreement (within 10%) with that measured using microscopy. It is observed that, with all other parameters kept fixed, the mean droplet size
of emulsions prepared with different percentage of dispersed phase. The data obtained from the optofluidic device and predicted by the correlation is in good agreement (within 10%) with that measured using microscopy. It is observed that, with all other parameters kept fixed, the mean droplet size  increases with the increase in the concentration of the dispersed phase [4].
increases with the increase in the concentration of the dispersed phase [4].
Figure 6. (a) Mean droplet size  for emulsion of varying sizes; (b) normalized DSD of an emulsion.
for emulsion of varying sizes; (b) normalized DSD of an emulsion.
Download figure:
Standard image High-resolution imageThe DSD of the emulsion was also measured using the optofluidic device. Figure 6(b) shows the size distribution of droplets in the emulsion with the dispersed phase of  in terms of normalized droplet count
in terms of normalized droplet count  versus droplet size
versus droplet size  . Here, for a particular droplet size, the count is normalized by the total count of droplets of all sizes. Data sets obtained from optical microscopy and the optofluidic device for each emulsion were separately fitted to normal distributions, as observed from figure 6(b), with
. Here, for a particular droplet size, the count is normalized by the total count of droplets of all sizes. Data sets obtained from optical microscopy and the optofluidic device for each emulsion were separately fitted to normal distributions, as observed from figure 6(b), with  0.88 and 0.99. The standard deviation
0.88 and 0.99. The standard deviation  of each normal distribution was considered to represent DSD (polydispersity) of emulsion. The standard deviations of the normal distribution curves with the data obtained from microscopy and optofluidic device are in good agreement (within 13%).
of each normal distribution was considered to represent DSD (polydispersity) of emulsion. The standard deviations of the normal distribution curves with the data obtained from microscopy and optofluidic device are in good agreement (within 13%).
The detection and measurement of monodispersed droplets generated inside microchannels have been reported [41, 42]. However, the measurement of mean droplet size  and DSD of a polydispersed emulsion in a microchannel have not received much attention. Here, we report the measurement of these characteristics of a polydispersed emulsions in a microchannel. Recently, measurement of the
and DSD of a polydispersed emulsion in a microchannel have not received much attention. Here, we report the measurement of these characteristics of a polydispersed emulsions in a microchannel. Recently, measurement of the  and DSD of polydispersed microparticles using complicated and expensive macroscopic acoustic attenuation spectroscopy (AAS) setup and its comparison with conventional microscopy setup was reported [23]. The comparisons showed 20% and 44% difference in
and DSD of polydispersed microparticles using complicated and expensive macroscopic acoustic attenuation spectroscopy (AAS) setup and its comparison with conventional microscopy setup was reported [23]. The comparisons showed 20% and 44% difference in  and DSD respectively between the AAS and microscopy data. In the present work, the difference between our optofluidics and microscopy data was found to be only 10% and 13% for
and DSD respectively between the AAS and microscopy data. In the present work, the difference between our optofluidics and microscopy data was found to be only 10% and 13% for  and DSD, respectively, which indicates the accuracy of our optofluidic system. This improvement can be attributed to the self-aligning characteristics of the droplets (alignment at the center due to lift forces) in the microchannels which minimizes the uncertainty in the position of the droplets and improves the consistency in the signal as explained in section 3.1.
and DSD, respectively, which indicates the accuracy of our optofluidic system. This improvement can be attributed to the self-aligning characteristics of the droplets (alignment at the center due to lift forces) in the microchannels which minimizes the uncertainty in the position of the droplets and improves the consistency in the signal as explained in section 3.1.
4. Conclusions
We presented an optofluidic device for in situ measurement of mean droplet size and DSD of an emulsion. First, the device was used to measure mean droplet size and frequency of droplets generated using an on-chip droplet generator. The droplet size and frequency was measured in terms of the magnitude and period of peak FSC. Droplets of different size were generated by varying the flow rates and characterized on a scatter plot in terms of forward scatter signal and the residence time in a detection window. Depending on the droplet size, distinct colonies were observed. The frequency of droplet generation was measured using the optofluidic device and microscopy and an excellent match was found (within 2%). Droplet size was then characterized in terms of the forward scatter signal and residence time. It was found that forward scatter signal increases nonlinearly with increase in droplet size while residence time was found to increase linearly with increase in droplet size. The residence time was correlated with droplet size which was then used for predicting the mean droplet size and DSD of an emulsion. The mean droplet size and DSD predicted using the correlation matches with that measured using microscopy within 10% and 13%, respectively. The proposed optofluidic device can be a useful tool for characterization of emulsions in chemical and biological applications.
Acknowledgments
The authors would like to thank SERB, DST, India (Project No. EMR/2014/001151) and IIT Madras for providing financial support for the project. We acknowledge the support of the Centre of NEMS and Nanophotonics (CNNP), IIT Madras with the photolithography work. Also, we thank the Interdisciplinary Research Program, IIT Madras, which enabled this work.